Physics Lournal
Powered by 🌱Roam GardenQED: Introduction
The focus of this lecture is a known part of physics, as opposed to an unknown part: people often question about the latest advancements in unification of theories, skipping over the theories that we understand deeply.
People question about things that are as of yet not understood, but rather than introduce you to theories that are currently being developed, we'll focus on one that has been thoroughly explored.
The area of physics in question is Quantum Electrodynamics, or the theory of light and matter, specifically the way light (photons) interact, and the nature of electrons.
Within the history of physics, many phenomena have been synthesized into a few theories. In the earlier history of physics, the phenomena of motion, heat, sound, light, and gravity were all separate, however the works of people like Newton, who gave us the laws of motion, showed us that things that appeared to be different were aspects of the same thing.
For example, sound is no longer separate from motion, and the phenomena of Heat can be understood in terms of motion.
Once motion, sound and heat were synthesized, we also discovered electrical and magnetic phenomena, which were synthesized with the phenomena of light and optics by Maxwell, by presenting light as an electromagnetic wave.
However, not everything has been handled in such a manner, as Gravity is not understandable in terms of other phenomena.
Circa 1900, a theory of matter emerged, based on the Electron, charged particles that existed inside of atoms, which evolved to include the Nucleus, around which the electrons orbited.
There was great difficulty in making sense of the Motion of electrons using mechanical laws in the manner Newton did, to explain how the earth revolved around the sun.
Developing another theory to subsume Newton's laws, because the behavior of matter at the atomic scale were unlike the behaviors at larger scales.
Making sense of what was taking place at the atomic scale required a revocation of common sense about how matter behaved.
In 1926, a theory to explain the new types of behavior observed in electrons, was developed, referred to as Quantum Mechanics.
This theory provided an explanation for many things, such as the reason for oxygen atoms combining with hydrogen atoms to make water, thus supplying the theory underlying chemistry.
Because this theory explain all of chemistry and the reason for the various properties of substances, it was hailed a success, but there was still no answer to the interaction between light and matter: Maxwell's Electrodynamics had to be altered to align with quantum theory, which began to be developed in 1929 by Dirac.
However, there were still issues with the theory, with it allowing for rough or approximate calculations, but introducing infinities as values for terms that were expected to be much smaller, putting a limit on the accuracy of possible calculations.
Dirac's work used relativity, to make a relativistic theory of the electron that didn't account for all of the ways that electrons interact with light.
His theory stated that an electron had a magnetic moment, with a strength of exactly in certain units, but later, around 1948, experimentalists measured this value to be with an uncertainty of , but when physicists attempted to calculate this value from the theory, the result was infinity, which didn't match up with experiment.
This discrepancy was cleared up by Julian Schwinger and Tomonaga, and the author, in 1948.
As it stands, QED has lasted over 50 years, being experimentally and theoretically verified with high precision.
When Newton observed light, he noticed that what is considered white light, is actually a combination of various colors of light, and showed how to separate light into these constituent colors with a prism, and furthermore, he noticed that a second prism didn't increase the separation of the various colors of light.
Technically, however, single colors of light can be further split according to the polarizations of the photons comprising the light.
It should also be noted that light does not just comprise the visible spectrum, but the entirety of the electromagnetic spectrum, from radio waves to gamma rays.
Newton made the assumption that light in fact was comprised of particles, which he termed corpuscles, and this fact has been verified by experiment.
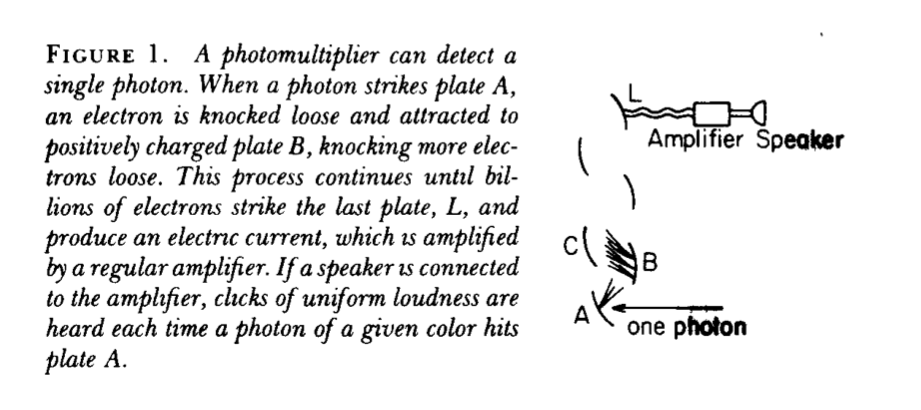
The detection of single photons is made possible by experimental apparatus such as the photomultiplier: